Respuestas
1.
a. (PH = -log ([H_3O ^ {+}]) = – log (1.218) = – 0.086 )
([HCl] = [H ^ {+}] )
(M_ {1} V_ {1} = M_ {2} V_ {2} rightarrow (6.09 , M) (25 , mL) = (x) (125 , mL) rightarrow x = 1.218 )
b. (pH + pOH = 14 pH de la derecha = 14-pOH = 14-0.80 = 13 )
(pOH = -log ([OH ^ {-}]) = – log (0.159) = 0.80 )
([NaOH] = [OH ^ {-}] )
(M_ {1} V_ {1} = M_ {2} V_ {2} rightarrow (2.55 , M) (5.0 , mL) = (x) (80 , mL) rightarrow x = 0.159 )
2.
(pH + pOH = 14 pH derecho = 14-pOH = 14-1.321 = 12.7 )
(HCl: 50.0 , mL times frac {1 , L} {1,000 , mL} times frac {0.225 , mol} {1 , L} = 0.01125 , mol )
(NaOH: 100.0 , mL times frac {1 , L} {1,000 , mL} times frac {0.184 , mol} {1 , L} = 0.0184 , mol )
Tenga en cuenta que el número de moles para (NaOH ) es mayor que (HCl ), por lo tanto, calculamos (pOH ).
(0.0184-0.01125 = 0.00715 , mol )
( frac {0.00715 , mol} {. 15 , L} = 0.0477 )
(pOH = -log ([OH ^ {-}]) = – log (0.0477) = 1.321 )
3. (M_ {1} V_ {1} = M_ {2} V_ {2} rightarrow (0.50 , M) (x , L) = (0.86 , M) (0.25 , L ) rightarrow x , L = 4.3 times 10 ^ {- 2} , L )
4. Solución de video
a. (pH = pK_a + log ( frac {C_ {2} H_ {3} NaO_ {2}} {C_ {2} H_ {4} O_ {2}}) = 4.76 + log ( frac {0.0935} { 0.0105}) = 5.71 )
(HCl: 100 , mL times frac {1 , L} {1,000 , mL} times frac {0.0105 , mol} {1 , L} = 0.0105 , mol )
(C_2H_3NaO_2: 100 , mL times frac {1 , L} {1,000 , mL} times frac {0.115 , mol} {1 , L} = 0.0115 , mol )
(C_ {2} H_ {3} NaO_ {2} , (s) + HCl , (aq) rightarrow C_ {2} H_ {4} O_ {2} , (aq) + NaCl , (s) )
|
(C_ {2} H_ {3} NaO_ {2} )
|
(HCl )
|
(C_ {2} H_ {4} O_ {2} )
|
(NaCl )
|
|
|
I
|
0,0115
|
0,0105
|
0
|
–
|
|
C
|
-0,0105
|
-0,0105
|
+0,0105
|
–
|
|
E
|
0,0935
|
0
|
0,0105
|
–
|
b. (pH = pK_a + log ( frac {C_ {2} H_ {3} NaO_ {2}} {C_ {2} H_ {4} O_ {2}}) = 4.76 + log ( frac {0.01} { 0.005}) = 5.05 )
(HCl: 100 , mL times frac {1 , L} {1,000 , mL} times frac {0.0105 , mol} {1 , L} = 0.005 , mol )
(C_2H_3NaO_2: 100 , mL times frac {1 , L} {1,000 , mL} times frac {0.115 , mol} {1 , L} = 0.015 , mol )
(C_ {2} H_ {3} NaO_ {2} , (s) + HCl , (aq) rightarrow C_ {2} H_ {4} O_ {2} , (aq) + NaCl , (s) )
|
(C_ {2} H_ {3} NaO_ {2} )
|
(HCl )
|
(C_ {2} H_ {4} O_ {2} )
|
(NaCl )
|
|
|
I
|
0,015
|
0,005
|
0
|
0
|
|
C
|
-0,005
|
-0,005
|
+0,005
|
+0,005
|
|
E
|
0,01
|
0
|
0,005
|
0,005
|
c. (pH = 14-pOH = 14 – (- log ( frac {0.0118-0.0109} {0.2 , L})) = 11.7 )
(CH_ {3} COOH: 0.1 , L veces 0.109 , M = 0.0109 , mol )
(NaOH: 0.1 , L veces 0.118 , M = 0.0118 , mol )
(CH_ {3} COOH , (aq) + NaOH , (aq) rightarrow H_ {2} O , (l) + CH_ {3} COO ^ {-} Na , (aq) )
|
(CH_ {3} COOH )
|
(NaOH )
|
(H_ {2} O )
|
(CH_ {3} COONa )
|
|
|
I
|
0,0109
|
0,0118
|
–
|
0
|
|
C
|
-0,0109
|
-0,0109
|
–
|
+0.0109
|
|
E
|
0
|
(9 veces 10 ^ {- 4} )
|
–
|
0,0109
|
d. (pH = 4.76 + log ( frac {0.0055} {0.0943}) = 3.53 )
(CH_ {3} COOH: 0.1 , L veces 0.998 , M = 0.0998 , mol )
(NaOH: 0.05 , L veces 0.110 , M = 0.0055 , mol )
(CH_ {3} COOH , (aq) + NaOH , (aq) rightarrow H_ {2} O , (l) + CH_ {3} COONa , (aq) )
|
(CH_ {3} COOH )
|
(NaOH )
|
(H_ {2} O )
|
(CH_ {3} COONa )
|
|
|
I
|
0,0998
|
0,0055
|
–
|
0
|
|
C
|
-0,0055
|
-0,0055
|
–
|
+0,0055
|
|
E
|
0,0943
|
0
|
–
|
0,0055
|
5. Solución de video
a. (pH = -log ( frac {0.9728} {0.2 , L}) = 4.86 )
(HCl: 100 , mL times frac {1 , L} {1,000 , L} times0.983 , M = 0.983 , mol )
(NaF: 100 , mL times frac {1 , L} {1,000 , L} times 0.102 , M = 0.0102 , mol )
(HCl , (aq) + NaF , (s) rightarrow NaCl , (aq) + HF , (aq) )
|
(HCl )
|
(NaF )
|
(NaCl )
|
(HF )
|
|
|
I
|
0,983
|
0,0102
|
–
|
0
|
|
C
|
-0,0102
|
-0,0102
|
–
|
-0,0102
|
|
E
|
0,9728
|
0
|
–
|
-0,0102
|
b. (pH = pK_a + log ( frac {[NaF]} {[HF]}) = 3.14 + log ( frac {0.00515} {0.00575}) = 3.09 )
(HCl: 50 , mL veces frac {1 , L} {1,000 , L} veces 0.115 , M = 0.00575 , mol )
(NaF: 100 , mL times frac {1 , L} {1,000 , L} times 0.109 , M = 0.0109 , mol )
(HCl , (aq) + NaF , (s) rightarrow NaCl , (aq) + HF , (aq) )
|
(HCl )
|
(NaF )
|
(NaCl )
|
(HF )
|
|
|
I
|
0,00575
|
0,0109
|
–
|
0
|
|
C
|
-0,00575
|
-0,00575
|
–
|
+0,00575
|
|
E
|
0
|
0,00515
|
–
|
0,00575
|
c. (pH = 14-pOH = 14 – (- log (0.0498-0.0106)) = 12.6 )
(HF: 100 , mL times frac {1 , L} {1,000 , L} times 0.106 , M = 0.0106 , mol )
(NaOH: 50 , mL veces frac {1 , L} {1,000 , L} veces 0.996 , M = 0.0498 , mol )
(HF , (aq) + NaOH , (aq) rightarrow NaF , (aq) + H_ {2} O , (aq) )
(HCl (aq) + NaOH (aq) NaCl derecho , (aq) + H_2O )
d. (PH = pK_a + log ( frac {[C_2H_3NaO_2]} {[CH_ {3} COOH]}) = 4.76 + log ( frac {0.0107} {0.04935}) = 4.10 ) [19459006 ]
(C_2H_3NaO_2: 0.1 , L veces 0.107 , M = 0.0107 )
(CH_3COOH: 0.05 , mL veces 0.987 , M = 0.04935 )
(CH_ {3} COOH , (aq) + H_ {2} O rightarrow C_2H_3O ^ {-} + H_3O ^ {+} )
6.
(CaCO_ {3} , (aq) +2 , HCl , (aq) rightarrow CaCl_ {2} , (aq) + H_2O , (l) + CO_ {2} , ( g) )
(CO_ {3} ^ {2 -} , (aq) + H ^ {+} , (aq) rightleftharpoons HCO_ {3} ^ {-} , (aq) )
(HCO_ {3} ^ {2 -} , (aq) + H ^ {+} , (aq) rightleftharpoons H_ {2} CO_ {3} , (aq) )
(K_ {a1} = 4.3 veces 10 ^ {- 7} )
(K_ {a2} = 5.0 veces 10 ^ {- 11} )
A 0 mL de adición de (HCl ): (pH = 14-pOH = 14 – (- log ( sqrt {0.0125 times frac {10 ^ {- 14}} {5.0 times 10 ^ {-11}}}) = 11.20 )
(CaCO_ {3}: 250 , mg times frac {1 , g} {1,000 , mg} times frac {1 , mol} {100.0869 , g} times frac {1} {200 , mL times frac {1 , L} {1,000 , mL}} = 0.0125 , M )
A 5 ml de adición de (HCl ): (pH = -log (5 times 10 ^ {- 11}) + log ( frac {0.002} {0.0005}) = 10.9 )
(CaCO_ {3}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.005 , L veces 0.1 , M = 0.0005 , mol )
|
(CO_ {3} ^ {2 -} )
|
(H ^ {+} )
|
(HCO_ {3} ^ {-} )
|
|
|
I
|
0,0025
|
0,0005
|
0
|
|
C
|
-0,0005
|
-0,0005
|
0,0005
|
|
E
|
0,002
|
0
|
0,0005
|
A 10 ml de adición de (HCl ): (pH = -log (5 times 10 ^ {- 11}) + log ( frac {0.001} {0.0015}) = 10.5 )
(CaCO_ {3}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.01 , L veces 0.1 , M = 0.001 , mol )
|
(CO_ {3} ^ {2 -} )
|
(H ^ {+} )
|
(HCO_ {3} ^ {-} )
|
|
|
I
|
0,0025
|
0,001
|
0
|
|
C
|
-0,001
|
-0,001
|
0,001
|
|
E
|
0,0015
|
0
|
0,001
|
Con 15 ml de adición de (HCl ): (pH = -log (5 times 10 ^ {- 11}) + log ( frac {0.001} {0.0015}) = 10.1 )
(CaCO_ {3}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.015 , L veces 0.1 , M = 0.0015 , mol )
|
(CO_ {3} ^ {2 -} )
|
(H ^ {+} )
|
(HCO_ {3} ^ {-} )
|
|
|
I
|
0,0025
|
0,0015
|
0
|
|
C
|
-0,0015
|
-0,0015
|
0,0015
|
|
E
|
0,001
|
0
|
0,0015
|
Con 20 ml de adición de (HCl ): (pH = -log (5 times 10 ^ {- 11}) + log ( frac {5 times 10 ^ {- 4}} {0.002} ) = 9.7 )
(CaCO_ {3}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.02 , L veces 0.1 , M = 0.002 , mol )
|
(CO_ {3} ^ {2 -} )
|
(H ^ {+} )
|
(HCO_ {3} ^ {-} )
|
|
|
I
|
0,0025
|
0,002
|
0
|
|
C
|
-0,002
|
-0,002
|
0,002
|
|
E
|
(5 veces 10 ^ {- 4} )
|
0
|
0,002
|
Con 25 ml de adición de (HCl ): primer punto de equivalencia: (pH = frac {-log (K_ {a1}) – log (K_ {a2})} {2} = frac {- log (4.3 times 10 ^ {- 7}) – log (5.0 times 10 ^ {- 11})} {2} = 8.33 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.025 , L veces 0.1 , M = 0.0025 , mol )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,0025
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0
|
0,0025
|
Con 30 ml de adición de (HCl ): (pH = -log (4.3 times 10 ^ {- 7}) + log ( frac {0.0025} {0.0005}) = 7.07 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.03 , L veces 0.1 , M = 0.003 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,003
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
(5 veces 10 ^ {- 4} )
|
0,0025
|
Con 35 ml de adición de (HCl ): (pH = -log (4.3 times 10 ^ {- 7}) + log ( frac {0.0025} {0.001}) = 6.76 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.035 , L veces 0.1 , M = 0.0035 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,0035
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,001
|
0,0025
|
A 40 mL de adición de (HCl ): (pH = -log (4.3 times 10 ^ {- 7}) + log ( frac {0.0025} {0.003}) = 6.59 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.040 , L veces 0.1 , M = 0.004 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,004
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,0015
|
0,0025
|
A 45 ml de adición de (HCl ): (pH = -log (4.3 times 10 ^ {- 7}) + log ( frac {0.0025} {0.002}) = 6.46 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.045 , L veces 0.1 , M = 0.0045 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,0045
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,002
|
0,0025
|
A 50 ml de adición de (HCl ): segundo punto de equivalencia: (pH = 3.86 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.05 , L veces 0.1 , M = 0.005 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,005
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,0025
|
0,0025
|
A 55 ml de adición de (HCl ): (pH = -log ( frac {0.003} {0.055}) = 1.26 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.055 , L veces 0.1 , M = 0.0055 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,0055
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,003
|
0,0025
|
A 60 ml de adición de (HCl ): (pH = -log ( frac {0.0035} {0.06}) = 1.23 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.06 , L veces 0.1 , M = 0.006 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,006
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,0035
|
0,0025
|
A 65 ml de adición de (HCl ): (pH = -log ( frac {0.0040} {0.065}) = 1.21 )
(HCO_ {3} ^ {-}: 0.2 , L veces 0.0125 , M = 0.0025 , mol )
(HCl: 0.06 , L veces 0.1 , M = 0.006 )
|
(HCO_ {3} ^ {-} )
|
(H ^ {+} )
|
(H_ {2} CO_ {3} )
|
|
|
I
|
0,0025
|
0,0065
|
0
|
|
C
|
-0,0025
|
-0,0025
|
0,0025
|
|
E
|
0
|
0,0040
|
0,0025
|
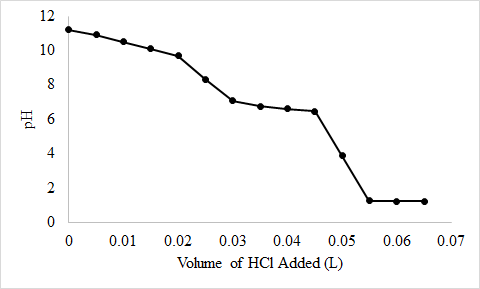
7. Solución de video
-
(M_1V_1 = M_2V_2 rightarrow V_2 = frac {M_1V_1} {V_2} = frac {(0.2 , M) (0.5 , L)} {12.0 , M} = 8.33 times10 ^ {- 3} , L ) Por lo tanto, diluya 8.33 mL de HCl 12.0 M a 500.0 mL.
-
(M_1V_1 = M_2V_2 rightarrow V_2 = frac {M_1V_1} {V_2} = frac {(0.288 , M) (0.05 , L)} {0.2 , M} = 7.20 veces 10 ^ { -3} , L )
-
(M_1V_1 = M_2V_2 rightarrow V_2 = frac {M_1V_1} {V_2} = frac {(0.288 , M) (0.05 , L)} {0.187 , M} = 7.70 veces 10 ^ { -2} )
8.
a. (M_ {1} V_ {1} = M_ {2} V_ {2} rightarrow M_2 = frac {M_1V_1} {V_ {2}} = frac {(0.582 , M) (0.060 , L) } {0.0719 , L} = 0.49 , M )
b. (M_ {1} V_ {1} = M_ {2} V_ {2} rightarrow V_ {2} = frac {M_ {1} V_ {1}} {M_ {2}} = frac {(0.582 , M) (0.050 , L)} {0.49 , M} = 59.4 veces 10 ^ {- 2} , L )
9.
| Volumen de base añadida (ml) | 10,0 | 30,0 | 40,0 | 45,0 | 50,0 | 55.0 | 65,0 | 75,0 |
| pH | 0,73 | 1,22 | 1,76 | 7 | 12,2 | 12,5 | 13,0 | 13,1 |
(NaOH , (aq) + HCl , (aq) rightarrow NaCl , (s) + H_ {2} O , (l) )
A 0 ml de base añadida: (pH = -log (0.288) = 0.54 )
A 10 ml de base añadida: (pH = -log ( frac {0.0144-0.00321} {0.06}) = 0.73 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.01 , L veces 0.321 , M = 0.00321 )
A 30 ml de base añadida: (pH = -log ( frac {0.0144-0.00963} {0.08}) = 1.22 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.03 , L veces 0.321 , M = 0.00963 )
A 40.0 mL de base agregada: (pH = -log ( frac {0.0144-0.01284} {0.09}) = 1.76 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.04 , L veces 0.321 , M = 0.01284 )
A 45,0 ml de base añadida: (pH = 7 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.045 , L veces 0.321 , M = 0.0144 )
Todo (HCl ) se neutralizará en el punto de equivalencia ([H ^ {+}] = [OH ^ {-}] ) y el pH de la solución es 7.
A 50.0 mL de base agregada: (pH = 14-pOH = 14-log ( frac {0.01605-0.0144} {0.1}) = 12.2 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.050 , L veces 0.321 , M = 0.01605 )
Con 55 ml de base añadida: (pH = 14-pOH = 14-log ( frac {0.01605-0.0144} {0.1}) = 12.5 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.055 , L veces 0.321 , M = 0.017655 )
A 65 ml de base añadida: (pH = 14-pOH = 14-log ( frac {0.020865-0.0144} {0.07}) = 13.0 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.065 , L veces 0.321 , M = 0.020865 )
A 75 ml de base añadida: (pH = 14-pOH = 14-log ( frac {0.024075-0.0144} {0.08}) = 13.1 )
(HCl: 0.05 , L veces 0.288 , M = 0.0144 )
(NaOH: 0.075 , L veces 0.321 , M = 0.024075 )
10.
El punto de equivalencia está en (pH ) 7 y esto ocurre en 0.0193 L.
(V_ {2} = frac {(0.156 , M) (0.025 , L)} {0.202} = 0.0193 , L )
11.
pH en el punto de equivalencia: (14 – (- log sqrt {( frac {10 ^ {- 14}} {10 ^ {- 3.75}} times frac {0.01205} {0.05 + 0.1227}} )) = 8.30 )
(0.05 , L veces 0.241 , M = 0.01205 , mol )
(V_2 = frac {(0.05 , L) (0.241 , M)} {0.0982} = 0.1227 , L )
| Volumen de base añadida (ml) | 0 | 5 | 10 | 15 | 20 | 25 |
| pH | 2,18 | 2,38 | 2,70 | 2,89 | 3,04 | 3.16 |
A 0 mL de base agregada: (pH = -log ([H ^ {+}]) = – log (2.52 times 10 ^ {- 6}) = 2.18 )
([H ^ {+}] = sqrt {10 ^ {- 3.75} times 0.241 , M} = 2.52 times 10 ^ {- 6} )
Con 5 ml de base añadida: (pH = 3.75 + log ( frac {4.91 times 10 ^ {- 4}} {0.01205-4.91 times 10 ^ {- 4}}) = 2.38 ) [ 19459006]
(KOH: 0.05 , L por 0.0982 , M = 4.91 por 10 ^ {- 4} , mol )
(CH_ {2} O_ {2}: 0.05 , L veces 0.241 , M = 0.01205 , mol )
Con 10 ml de base añadida: (pH = 3.75 + log ( frac {9.82 times 10 ^ {- 4}} {0.01205-9.82 times 10 ^ {- 4}}) = 2.70 ) [ 19459006]
(KOH: 0.01 , L veces 0.0982 , M = 9.82 veces 10 ^ {- 4} , mol )
(CH_ {2} O_ {2}: 0.05 , L veces 0.241 , M = 0.01205 , mol )
Con 15 ml de base añadida: (pH = 3.75 + log ( frac {0.001473} {0.01205-0.001473} = 2.89 )
(KOH: 0.015 , L veces 0.0982 , M = 0.001473 )
(CH_ {2} O_ {2}: 0.05 , L veces 0.241 , M = 0.01205 )
A 20 ml de base añadida: (pH = 3.75 + log ( frac {0.001964} {0.01205-0.001964}) = 3.04 )
(KOH: 0.02 , L veces 0.0982 , M = 0.001964 )
(CH_ {2} O_ {2}: 0.05 , L veces 0.241 , M = 0.01205 )
Con 25 ml de base añadida: (pH = 3.75 + log ( frac {0.002455} {0.01205-0.002455}) = 3.16 )
(KOH: 0.025 , L veces 0.0982 , M = 0.002455 , mol )
(CH_ {2} O_ {2}: 0.05 , L veces 0.241 , M = 0.01205 )
12. (M_1V_1 = M_2V_2 rightarrow V_2 = frac {M_1V1} {M_2} = frac {(0.430 , M) (0.05 , L)} {0.150 , M} = 1.42 veces 10 ^ {- 1} , L )
Se necesitan 143 ml para desprotonar completamente el grupo ácido carboxílico.
a. Se necesitan 143 mililitros adicionales de KOH para desprotonar el grupo amonio.
b. En el primer punto de equivalencia: (pH = frac {(pKa_ {1} + pKa_ {2})} {2} = 5.95 )
c. Se necesitan 143 ml de titulante para obtener una solución en la que la glicina no tiene carga eléctrica. El punto isoeléctrico de la glicina 5.95.
13.
(pH = pK_a + log ( frac {[C_5H_5N]} {[C_5H_5NH ^ {+}]}) = (14-8.77) + log ( frac {0.02996} {0.0063434}) = 5.90 )
(C_5H_5N , (aq) + HCl , (aq) rightarrow C_5H_5NH ^ {+} , (aq) + Cl ^ {-} , (aq) )
(HCl: 32.2 , mL times frac {1 , L} {1,000 , mL} times 0.197 , M = 0.0063434 , mol )
(C_5H_5N: 150 , mL times frac {1 , L} {1,000 , mL} times 0.242 , M = 0.0363 , mol )
|
(C_5H_5N )
|
(HCl )
|
(C_5H_5NH ^ {+} )
|
(Cl ^ {-} )
|
|
|
I
|
0,0363
|
0,0063434
|
0
|
–
|
|
C
|
-0,0063434
|
-0,0063434
|
+0,0063434
|
–
|
|
E
|
0,02996
|
0
|
0,0063434
|
–
|
14. (pH = frac {(4.21 + 5.64)} {2} = 4.93 )
15. (pH = frac {(2.85 + 5.70)} {2} = 4.25 )