Estos son ejercicios de tarea para acompañar el Mapa de texto creado para “Química: la ciencia central” por Brown et al. Se pueden encontrar bancos de preguntas de química general complementaria para otros mapas de texto y se puede acceder aquí . Además de estas preguntas disponibles públicamente, el acceso al banco privado de problemas para su uso en exámenes y tareas está disponible para los profesores solo de manera individual; comuníquese con Delmar Larsen para obtener una cuenta con permiso de acceso.
2.1: La teoría atómica de la materia
Conceptual Problemas
-
¿Cuál de los siguientes elementos existen como moléculas diatómicas?
- helio
- hidrógeno
- yodo
- oro
-
¿Cuál de los siguientes elementos existen como moléculas diatómicas?
- cloro
- potasio
- plata
- oxígeno
-
¿Por qué es apropiado representar la forma elemental de helio como He pero impropio representar la forma elemental de hidrógeno como H?
-
¿Por qué es apropiado representar la forma elemental de cloro como Cl 2 pero impropio representar la forma elemental de calcio como Ca 2 ?
Conceptual Solución s
-
- no
- sí
- sí
- no
-
El hidrógeno existe como una molécula diatómica en su forma elemental; El helio no existe como molécula diatómica.
Ejercicios
-
¿Cuál de los siguientes elementos existen como moléculas diatómicas?
- helio
- hidrógeno
- yodo
- oro
-
¿Cuál de los siguientes elementos existen como moléculas diatómicas?
- cloro
- potasio
- plata
- oxígeno
-
¿Por qué es apropiado representar la forma elemental de helio como He pero impropio representar la forma elemental de hidrógeno como H?
-
¿Por qué es apropiado representar la forma elemental de cloro como Cl 2 pero impropio representar la forma elemental de calcio como Ca 2 ?
Respuestas
-
- no
- sí
- sí
- no
2.2: El descubrimiento de la estructura atómica
Conceptual Ejercicios
- ¿Qué es la teoría atómica moderna?
- ¿Qué son los átomos?
Conceptual Respuestas
- La teoría atómica moderna establece que toda la materia está compuesta de átomos.
- Los átomos son las partes más pequeñas de un elemento que mantienen la identidad de ese elemento.
Problemas numéricos
- (Verificación del concepto básico) Cuando se queman 32.0 gramos (g) de metano en 128.0 g de oxígeno, se producen 88.0 g de dióxido de carbono y 72.0 g de agua. ¿De qué ley es este un ejemplo? (a) Ley de proporciones definidas (b) Ley de conservación de la masa o (c) Ley de proporciones múltiples.
- (Ley de conservación de la masa) Se queman 8.00 gramos (g) de metano en 32.00 g de oxígeno. La reacción produce 22.00 g de dióxido de carbono y una masa de agua no medida. ¿Qué masa de agua se produce?
- (Ley de proporciones definidas) Se realizan dos experimentos con sodio y cloro. En el primer experimento, se hacen reaccionar 4,36 gramos (g) de sodio con 32,24 g de cloro, utilizando todo el sodio. Se produjeron 11,08 g de cloruro de sodio en el primer experimento. En el segundo experimento, 4.20 g de cloro reaccionaron con 20.00 g de sodio, utilizando todo el cloro. Se produjeron 6,92 g de cloruro de sodio en el segundo experimento. Muestre que estos resultados son consistentes con la ley de composición constante.
- (Ley de conservación de la masa): 36.0 gramos (g) de madera se queman en oxígeno. Los productos de esta reacción pesan 74,4 g. (a) ¿Qué masa de oxígeno se necesita en esta reacción? (b) ¿Qué masa de oxígeno se necesita para quemar 8.00 lb de madera? 1 libra = 453.59237 g.
- (Ley de proporciones definidas): una muestra de metano contiene solo carbono e hidrógeno, con 3.00 gramos (g) de carbono por cada 1.00 g de hidrógeno. ¿Cuánto hidrógeno debería estar presente en un 50.0 g diferente de metano?
Soluciones numéricas
- La respuesta es (b) Ley de conservación de la masa. La cantidad de gramos de reactivos (32.0 g de metano y 128.0 g de oxígeno = 160.0 g en total) es igual a la cantidad de gramos de producto (88.0 g de dióxido de carbono y 72.0 g de agua = 160.0 g en total).
- La respuesta es 18.00 g de agua. Debido a que los únicos productos son agua y dióxido de carbono, su masa total debe ser igual a la masa total de los reactivos, metano y oxígeno. 8,00 g de metano + 32,00 g de oxígeno = 40,00 g totales de reactivos. Debido a que la masa total de los reactivos es igual a la masa total de los productos, la masa total de los productos también es 40.00 g. Por lo tanto, 40.00 g totales de productos = 22.00 g de dióxido de carbono + masa de agua desconocida. 40.00 g de productos totales – 22.00 g de dióxido de carbono = 18.00 g de agua.
-
Para resolver, determine el porcentaje de sodio en cada muestra de cloruro de sodio. Hay 4,36 g de sodio por cada 11,08 g de cloruro de sodio en el primer experimento. The amount of sodium in the sodium chloride for the second experiment must be found. This is found by subtracted the known amount of reacted chlorine (4.20 g) from the amount of sodium chloride (6.92 g). 6.92 g sodium chloride – 4.20 g chlorine = 2.72 g sodium.
Thus, the percent of sodium in each sample is represented below:
% Na = (4.36 g Na)/(11.08 g NaCl) x 100% = 39.4% Na % Na = (2.72 g Na)/(6.92 g NaCl) x 100% = 39.3%
The slight difference in compositions is due to significant figures: each percent has an uncertainty of .01% in either direction. The two samples of sodium chloride have the same composition. -
- The answer is 38.4 g of oxygen. The total mass of the products is 74.4 g. Thus, the total mass of the reactants must equal 74.4 g as well. Thus, 74.4 g products – 36.0 g wood reactant = 38.4 g oxygen reactant.
- The answer is 8.53 lb of oxygen. From, (a) that it takes 38.4 g of oxygen to burn 18.0 g of wood. First, convert both of these values to pounds (alternatively, the 8.00 lb can be converted to grams).
36.0 g wood x (1lb)/(453.59237 g) = 0.0793664144 lb wood
38.4 g oxygen x (1 lb)/(453.59237 g) = .0846575087 lb oxygen
Now two ratios equal to each other can be set up to determine the unknown mass of oxygen.
(0.0793664144 lb wood)/(.0846575087 lb oxygen) = (8.00 lb wood)/(unknown mass oxygen)
Solving reveals that it requires 8.53 lb of oxygen to burn 8.00 lb of wood.
- The answer is 12.5 g of hydrogen. If there are 3.00 g of carbon present for every 1.00 g of hydrogen, we can assume the smallest whole number combination of these elements in that ratio to be 4.00 g of methane:
50.0 g methane x (1.00 g hydrogen)/(4.00 g methane) = 12.5 g of hydrogen.
2.3: The Modern View of Atomic Structure
Conceptual Problems
- Describe the experiment that provided evidence that the proton is positively charged.
- What observation led Rutherford to propose the existence of the neutron?
- What is the difference between Rutherford’s model of the atom and the model chemists use today?
- If cathode rays are not deflected when they pass through a region of space, what does this imply about the presence or absence of a magnetic field perpendicular to the path of the rays in that region?
- Describe the outcome that would be expected from Rutherford’s experiment if the charge on α particles had remained the same but the nucleus were negatively charged. If the nucleus were neutral, what would have been the outcome?
- Describe the differences between an α particle, a β particle, and a γ ray. Which has the greatest ability to penetrate matter?
Numerical Problems
Please be sure you are familiar with the topics discussed in Section 1.6 before proceeding to the Numerical Problems.
- Using the data in Table 1.3 and the periodic table, calculate the percentage of the mass of a silicon atom that is due to
- electrons.
- protons.
- Using the data in Table 1.3 and the periodic table, calculate the percentage of the mass of a helium atom that is due to
- electrons.
- protons.
- The radius of an atom is approximately 10 4 times larger than the radius of its nucleus. If the radius of the nucleus were 1.0 cm, what would be the radius of the atom in centimeters? in miles?
- The total charge on an oil drop was found to be 3.84 × 10 −18 coulombs. What is the total number of electrons contained in the drop?
2.4: Atomic Mass
Conceptual Problems
1. Complete the following table for the missing elements, symbols, and numbers of electrons.
| Element | Symbol | Number of Electrons |
| molybdenum | ||
| 19 | ||
| titanium | ||
| B | ||
| 53 | ||
| Sm | ||
| helium | ||
| 14 |
2. Complete the following table for the missing elements, symbols, and numbers of electrons.
| Element | Symbol | Number of Electrons |
| lanthanum | ||
| Ir | ||
| aluminum | ||
| 80 | ||
| sodium | ||
| Si | ||
| 9 | ||
| Be |
3. Is the mass of an ion the same as the mass of its parent atom? Explain your answer.
4. What isotopic standard is used for determining the mass of an atom?
5. Give the symbol
- beryllium
- ruthenium
- phosphorus
- aluminum
- cesium
- praseodymium
- cobalt
- yttrium
- arsenic
6. Give the symbol
- fluorine
- helium
- terbium
- iodine
- gold
- scandium
- sodium
- niobium
- manganese
7. Identify each element, represented by X, that have the given symbols.
-
(_{26}^{55}X) -
(_{33}^{74}X) -
(_{12}^{24}X) -
(_{53}^{127}X) -
(_{18}^{40}X) -
(_{63}^{152}X)
Numerical Problems
Please be sure you are familiar with the topics discussed in Section 1.6 before proceeding to the Numerical Problems.
1. The isotopes 131 I and 60 Co are commonly used in medicine. Determine the number of neutrons, protons, and electrons in a neutral atom of each.
2. Determine the number of protons, neutrons, and electrons in a neutral atom of each isotope:
- (^{97}Tc)
- (^{113}In)
- (^{63}Ni)
- (^{55}Fe)
3. Both technetium-97 and americium-240 are produced in nuclear reactors. Determine the number of protons, neutrons, and electrons in the neutral atoms of each.
4. The following isotopes are important in archaeological research. How many protons, neutrons, and electrons does a neutral atom of each contain?
- (^{207}Pb)
- (^{16}O)
- (^{40}K)
- (^{137}Cs)
- (^{40}Ar)
5. Copper, an excellent conductor of heat, has two isotopes: 63 Cu and 65 Cu. Use the following information to calculate the average atomic mass of copper:
| Isotope | Percent Abundance (%) | Atomic Mass (amu) |
| 63 Cu | 69.09 | 62.9298 |
| 65 Cu | 30.92 | 64.9278 |
6. Silicon consists of three isotopes with the following percent abundances:
| Isotope | Percent Abundance (%) | Atomic Mass (amu) |
| 28 Si | 92.18 | 27.976926 |
| 29 Si | 4.71 | 28.976495 |
| 30 Si | 3.12 | 29.973770 |
Calculate the average atomic mass of silicon.
7. Complete the following table for neon. The average atomic mass of neon is 20.1797 amu.
| Isotope | Percent Abundance (%) | Atomic Mass (amu) |
| 20 Ne | 90.92 | 19.99244 |
| 21 Ne | 0.257 | 20.99395 |
| 22 Ne |
8. Are
9.Complete the following table:
| Isotope | Number of Protons | Number of Neutrons | Number of Electrons |
| 238 X | 95 | ||
| 238 U | |||
| 75 | 112 |
10.Complete the following table:
| Isotope | Number of Protons | Number of Neutrons | Number of Electrons |
| 57 Fe | |||
| 40 X | 20 | ||
| 36 S |
11. Using a mass spectrometer, a scientist determined the percent abundances of the isotopes of sulfur to be 95.27% for 32 S, 0.51% for 33 S, and 4.22% for 34 S. Use the atomic mass of sulfur from the periodic table (see Chapter 32 “Appendix H: Periodic Table of Elements” ) and the following atomic masses to determine whether these data are accurate, assuming that these are the only isotopes of sulfur: 31.972071 amu for 32 S, 32.971459 amu for 33 S, and 33.967867 amu for 34 S.
12. The percent abundances of two of the three isotopes of oxygen are 99.76% for 16 O, and 0.204% for 18 O. Use the atomic mass of oxygen given in the periodic table and the following data to determine the mass of 17 O: 15.994915 amu for 16 O and 17.999160 amu for 18 O.
13. Which element has the higher proportion by mass in NaI?
14. Which element has the higher proportion by mass in KBr?
2.5: The Periodic Table
Conceptual Problems
1. Classify each element in Conceptual Problem 1 as a metal, a nonmetal, or a semimetal. If a metal, state whether it is an alkali metal, an alkaline earth metal, or a transition metal.
2. Classify each element in Conceptual Problem 2) as a metal, a nonmetal, or a semimetal. If a metal, state whether it is an alkali metal, an alkaline earth metal, or a transition metal.
3. Classify each element as a metal, a semimetal, or a nonmetal. If a metal, state whether it is an alkali metal, an alkaline earth metal, or a transition metal.
- iron
- tantalum
- sulfur
- silicon
- chlorine
- nickel
- potassium
- radon
- zirconium
4. Which of these sets of elements are all in the same period?
- potassium, vanadium, and ruthenium
- lithium, carbon, and chlorine
- sodium, magnesium, and sulfur
- chromium, nickel, and krypton
5. Which of these sets of elements are all in the same period?
- barium, tungsten, and argon
- yttrium, zirconium, and selenium
- potassium, calcium, and zinc
- scandium, bromine, and manganese
6. Which of these sets of elements are all in the same group?
- sodium, rubidium, and barium
- nitrogen, phosphorus, and bismuth
- copper, silver, and gold
- magnesium, strontium, and samarium
7. Which of these sets of elements are all in the same group?
- iron, ruthenium, and osmium
- nickel, palladium, and lead
- iodine, fluorine, and oxygen
- boron, aluminum, and gallium
8. Indicate whether each element is a transition metal, a halogen, or a noble gas.
- manganese
- iridium
- fluorine
- xenon
- lithium
- carbon
- zinc
- sodium
- tantalum
- hafnium
- antimony
- cadmium
9. Which of the elements indicated in color in the periodic table shown below is most likely to exist as a monoatomic gas? As a diatomic gas? Which is most likely to be a semimetal? A reactive metal?
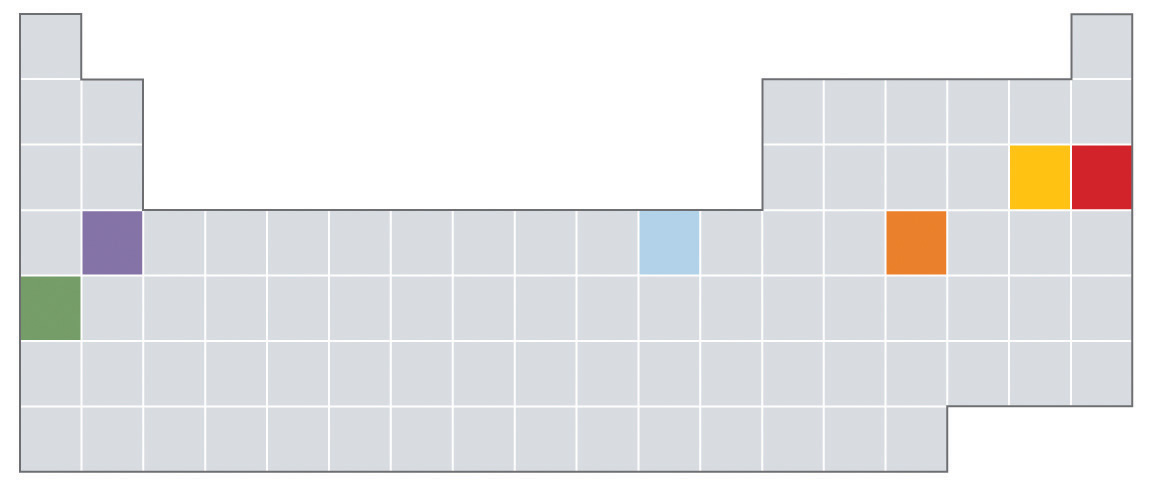
10. Based on their locations in the periodic table, would you expect these elements to be malleable? ¿Por qué o por qué no?
- phosphorus
- chromium
- rubidium
- copper
- aluminum
- bismuth
- neodymium
11. Based on their locations in the periodic table, would you expect these elements to be lustrous? ¿Por qué o por qué no?
- sulfur
- vanadium
- nickel
- arsenic
- strontium
- cerium
- sodium
Conceptual Solution
3.
| Symbol | Type |
|---|---|
| Fe | metal: transition metal |
| Ta | metal: transition metal |
| S | nonmetal |
| Si | semimetal |
| Cl | nonmetal (halogen) |
| Ni | metal: transition metal |
| K | metal: alkali metal |
| Rn | nonmetal (noble gas) |
| Zr | metal: transition meta |
2.6: Molecules and Molecular Compounds
Conceptual Problems
- Ionic and covalent compounds are held together by electrostatic attractions between oppositely charged particles. Describe the differences in the nature of the attractions in ionic and covalent compounds. Which class of compounds contains pairs of electrons shared between bonded atoms?
- Which contains fewer electrons than the neutral atom—the corresponding cation or the anion?
- What is the difference between an organic compound and an inorganic compound?
- What is the advantage of writing a structural formula as a condensed formula?
- The majority of elements that exist as diatomic molecules are found in one group of the periodic table. Identify the group.
- Discuss the differences between covalent and ionic compounds with regard to
- a. the forces that hold the atoms together.
- b. melting points.
- c. physical states at room temperature and pressure.
- Why do covalent compounds generally tend to have lower melting points than ionic compounds?
Conceptual Answer
7. Covalent compounds generally melt at lower temperatures than ionic compounds because the intermolecular interactions that hold the molecules together in a molecular solid are weaker than the electrostatic attractions that hold oppositely charged ions together in an ionic solid.
Numerical Problems
1. The structural formula for chloroform (CHCl 3 ) was shown in Example 2.6.2. Based on this information, draw the structural formula of dichloromethane (CH 2 Cl 2 ).
2. What is the total number of electrons present in each ion?
- F −
- Rb +
- Ce 3 +
- Zr 4 +
- Zn 2 +
- Kr 2 +
- B 3 +
3. What is the total number of electrons present in each ion?
- Ca 2 +
- Se 2−
- In 3 +
- Sr 2 +
- As 3 +
- N 3−
- Tl +
4. Predict how many electrons are in each ion.
- an oxygen ion with a −2 charge
- a beryllium ion with a +2 charge
- a silver ion with a +1 charge
- a selenium ion with a +4 charge
- an iron ion with a +2 charge
- a chlorine ion with a −1 charge
5. Predict how many electrons are in each ion.
- copper ion with a +2 charge
- a molybdenum ion with a +4 charge
- an iodine ion with a −1 charge
- a gallium ion with a +3 charge
- an ytterbium ion with a +3 charge
- a scandium ion with a +3 charge
6. Predict the charge on the most common monatomic ion formed by each element.
- chlorine
- phosphorus
- scandium
- magnesium
- arsenic
- oxygen
7. Predict the charge on the most common monatomic ion formed by each element.
- sodium
- selenium
- barium
- rubidium
- nitrogen
- aluminum
8. For each representation of a monatomic ion, identify the parent atom, write the formula of the ion using an appropriate superscript, and indicate the period and group of the periodic table in which the element is found.
- (_4^9X^{2+} )
- (_1^1X^-)
- (_8^{16}X^{2-} )
9. For each representation of a monatomic ion, identify the parent atom, write the formula of the ion using an appropriate superscript, and indicate the period and group of the periodic table in which the element is found.
- (_3^7X^+ )
- (_9^{19}X^-)
- (_{13}^{27}X^{3+})
Numerical Answers
5.
- 27
- 38
- 54
- 28
- 67
- 18
9.
- Li, Li + , 2nd period, group 1
- F, F – , 2nd period, group 17
- Al, Al 3 + , 3nd period, group 13
2.7: Ions and Ionic Compounds
2.8: Naming Inorganic Compounds
Conceptual Problems
1. What are the differences and similarities between a polyatomic ion and a molecule?
2. Classify each compound as ionic or covalent.
- Zn 3 (PO 4 ) 2
- C 6 H 5 CO 2 H
- K 2 Cr 2 O 7
- CH 3 CH 2 SH
- NH 4 Br
- CCl 2 F 2
3. Classify each compound as ionic or covalent. Which are organic compounds and which are inorganic compounds?
- CH 3 CH 2 CO 2 H
- CaCl 2
- Y(NO 3 ) 3
- H 2 S
- NaC 2 H 3 O 2
4. Generally, one cannot determine the molecular formula directly from an empirical formula. What other information is needed?
5. Give two pieces of information that we obtain from a structural formula that we cannot obtain from an empirical formula.
6. The formulas of alcohols are often written as ROH rather than as empirical formulas. For example, methanol is generally written as CH 3 OH rather than CH 4 O. Explain why the ROH notation is preferred.
7. The compound dimethyl sulfide has the empirical formula C 2 H 6 S and the structural formula CH 3 SCH 3 . What information do we obtain from the structural formula that we do not get from the empirical formula? Write the condensed structural formula for the compound.
8. What is the correct formula for magnesium hydroxide—MgOH 2 or Mg(OH) 2 ? ¿Por qué?
9. Magnesium cyanide is written as Mg(CN) 2 , not MgCN 2 . ¿Por qué?
10. Does a given hydrate always contain the same number of waters of hydration?
Conceptual Solutions
Numerical Problems
1. Write the formula for each compound.
- magnesium sulfate, which has 1 magnesium atom, 4 oxygen atoms, and 1 sulfur atom
- ethylene glycol (antifreeze), which has 6 hydrogen atoms, 2 carbon atoms, and 2 oxygen atoms
- acetic acid, which has 2 oxygen atoms, 2 carbon atoms, and 4 hydrogen atoms
- potassium chlorate, which has 1 chlorine atom, 1 potassium atom, and 3 oxygen atoms
- sodium hypochlorite pentahydrate, which has 1 chlorine atom, 1 sodium atom, 6 oxygen atoms, and 10 hydrogen atoms
2. Write the formula for each compound.
- cadmium acetate, which has 1 cadmium atom, 4 oxygen atoms, 4 carbon atoms, and 6 hydrogen atoms
- barium cyanide, which has 1 barium atom, 2 carbon atoms, and 2 nitrogen atoms
- iron(III) phosphate dihydrate, which has 1 iron atom, 1 phosphorus atom, 6 oxygen atoms, and 4 hydrogen atoms
- manganese(II) nitrate hexahydrate, which has 1 manganese atom, 12 hydrogen atoms, 12 oxygen atoms, and 2 nitrogen atoms
- silver phosphate, which has 1 phosphorus atom, 3 silver atoms, and 4 oxygen atoms
3. Complete the following table by filling in the formula for the ionic compound formed by each cation-anion pair.
| Ion | K + | Fe 3 + | NH 4 + | Ba 2 + |
|---|---|---|---|---|
| Cl − | KCl | |||
| SO 4 2− | ||||
| PO 4 3− | ||||
| NO 3 − | ||||
| OH − |
4. Write the empirical formula for the binary compound formed by the most common monatomic ions formed by each pair of elements.
- zinc and sulfur
- barium and iodine
- magnesium and chlorine
- silicon and oxygen
- sodium and sulfur
5. Write the empirical formula for the binary compound formed by the most common monatomic ions formed by each pair of elements.
- lithium and nitrogen
- cesium and chlorine
- germanium and oxygen
- rubidium and sulfur
- arsenic and sodium
6. Write the empirical formula for each compound.
- Na 2 S 2 O 4
- B 2 H 6
- C 6 H 12 O 6
- P 4 O 10
- KMnO 4
7. Write the empirical formula for each compound.
- Al 2 Cl 6
- K 2 Cr 2 O 7
- C 2 H 4
- (NH 2 ) 2 CNH
- CH 3 COOH
Numerical Answers
1.
- MgSO 4
- C 2 H 6 O 2
- C 2 H 4 O 2
- KClO 3
- NaOCl·5H 2 O
3.
| Ion | K + | Fe 3+ | NH 4 + | Ba 2+ |
|---|---|---|---|---|
| Cl − | KCl | FeCl 3 | NH 4 Cl | BaCl 2 |
| SO 4 2− | K 2 SO 4 | Fe2(SO4) 3 | (NH 4 ) 2 SO 4 | BaSO 4 |
| PO 4 3 − | K 3 PO 4 | FePO 4 | (NH 4 )3PO 4 | Ba3(PO 4 ) 2 |
| NO 3 − | KNO 3 | Fe(NO3) 3 | NH4NO 3 | Ba(NO 3 ) 2 |
| OH − | KOH | Fe(OH) 3 | NH 4 OH | Ba(OH) 2 |
5.
- Li 3 N
- CsCl
- GeO 2
- Rb 2 S
- Na 3 As
7.
- AlCl 3
- K 2 Cr 2 O 7
- CH 2
- CH 5 N 3
- CH 2 O
2.9: Some Simple Organic Compounds
Conceptual Problems
1. Benzene (C 6 H 6 ) is an organic compound, and KCl is an ionic compound. The sum of the masses of the atoms in each empirical formula is approximately the same. How would you expect the two to compare with regard to each of the following? What species are present in benzene vapor?
- melting point
- type of bonding
- rate of evaporation
- structure
2. Can an inorganic compound be classified as a hydrocarbon? ¿Por qué o por qué no?
3. Is the compound NaHCO 3 a hydrocarbon? ¿Por qué o por qué no?
4. Name each compound.
- NiO
- TiO 2
- N 2 O
- CS 2
- SO 3
- NF 3
- SF 6
5. Name each compound.
- HgCl 2
- IF 5
- N 2 O 5
- Cl 2 O
- HgS
- PCl 5
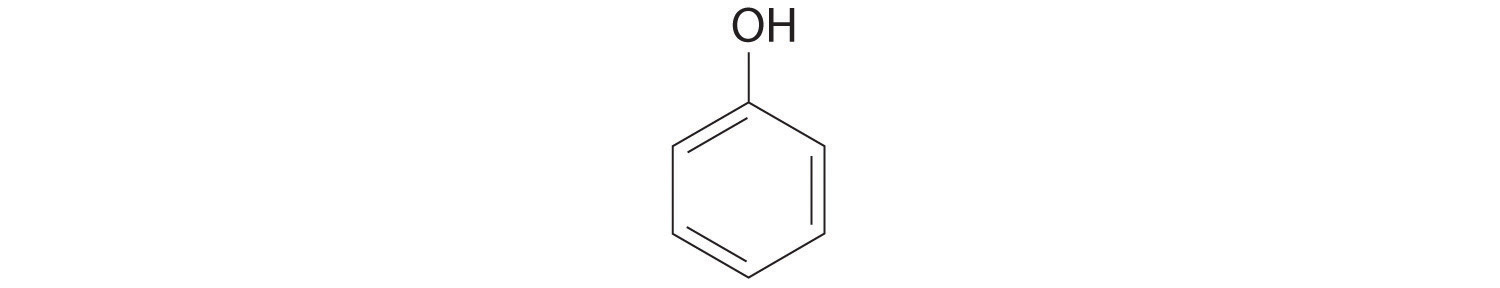
6. For each structural formula, write the condensed formula and the name of the compound.
a.
b.
c.
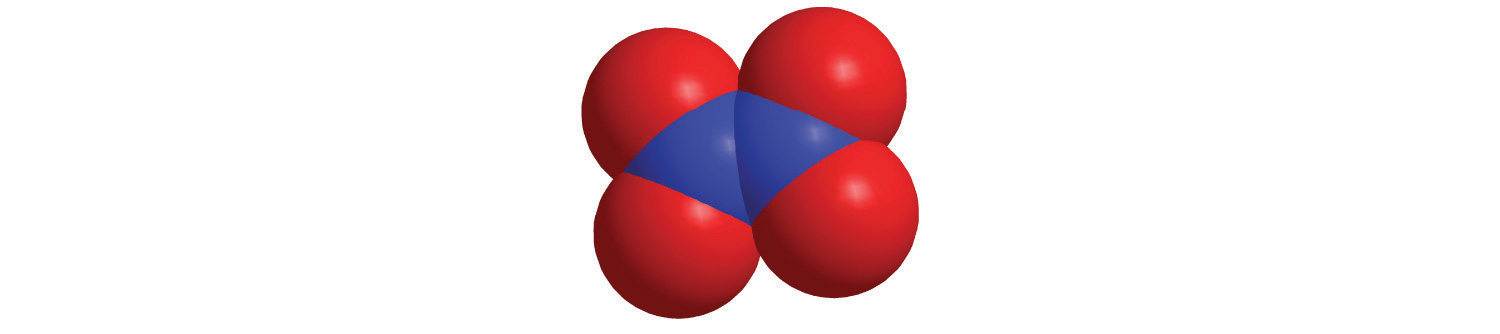
d.
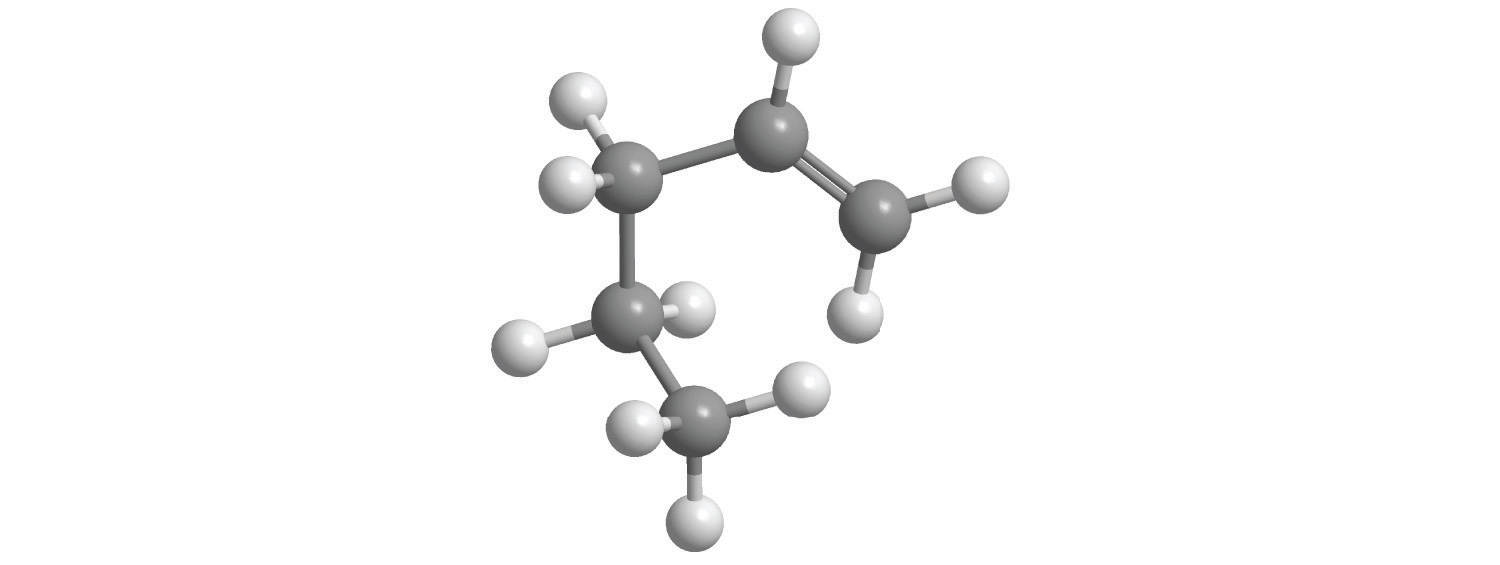
e.
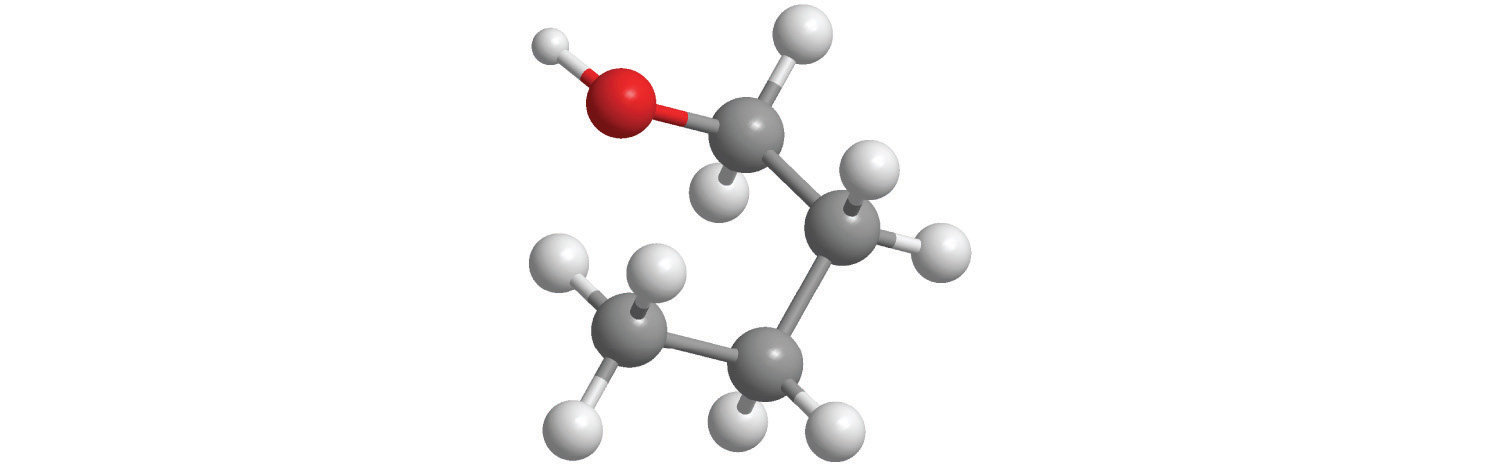
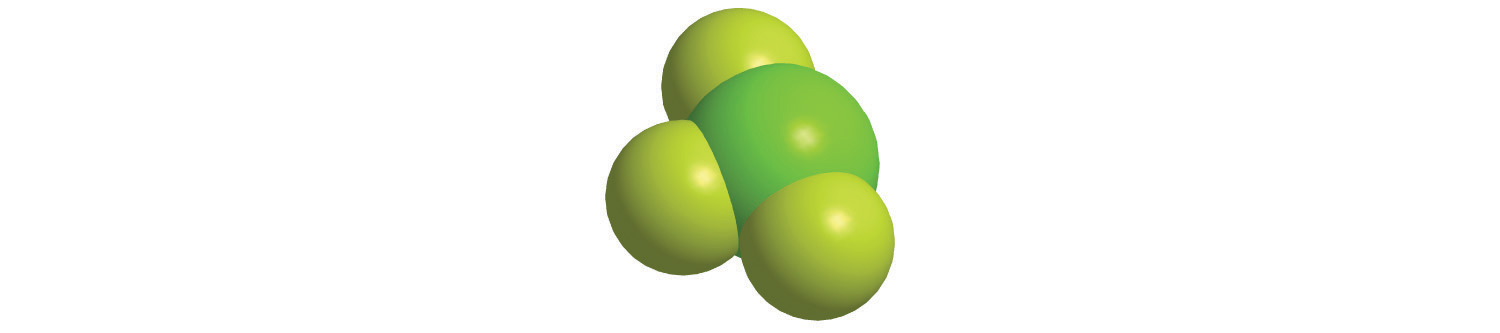
7. For each structural formula, write the condensed formula and the name of the compound.
a.
b.

c.
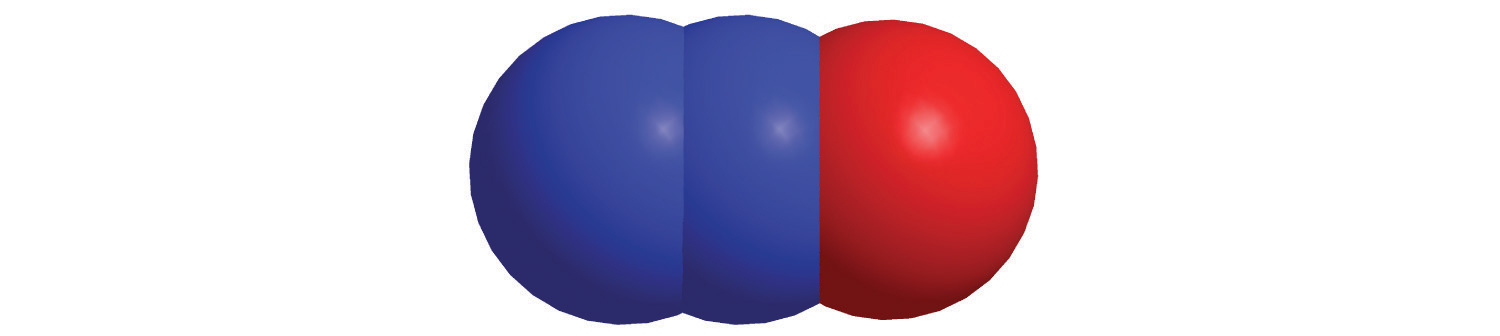
d.

8. Would you expect PCl 3 to be an ionic compound or a covalent compound? Explain your reasoning.
9. What distinguishes an aromatic hydrocarbon from an aliphatic hydrocarbon?
10. The following general formulas represent specific classes of hydrocarbons. Refer to Table 2.7 “The First 10 Straight-Chain Alkanes” and Table 2.8 “Some Common Acids That Do Not Contain Oxygen” and Figure 2.16 and identify the classes.
- C n H 2n + 2
- C n H 2n
- C n H 2n − 2
11. Using R to represent an alkyl or aryl group, show the general structure of an
- alcohol.
- phenol.
Conceptual Answer
11.
- ROH (where R is an alkyl group)
- ROH (where R is an aryl group)
Numerical Problems
1. Write the formula for each compound.
- dinitrogen monoxide
- silicon tetrafluoride
- boron trichloride
- nitrogen trifluoride
- phosphorus tribromide
2. Write the formula for each compound.
- dinitrogen trioxide
- iodine pentafluoride
- boron tribromide
- oxygen difluoride
- arsenic trichloride
3. Write the formula for each compound.
- thallium(I) selenide
- neptunium(IV) oxide
- iron(II) sulfide
- copper(I) cyanide
- nitrogen trichloride
4. Name each compound.
- RuO 4
- PbO 2
- MoF 6
- Hg 2 (NO 3 ) 2 ·2H 2 O
- WCl 4
5. Name each compound.
- NbO 2
- MoS2
- P 4 S 10
- Cu 2 O
- ReF 5
6. Draw the structure of each compound.
- propyne
- ethanol
- n-hexane
- cyclopropane
- benzene
7. Draw the structure of each compound.
- 1-butene
- 2-pentyne
- cycloheptane
- toluene
- phenol
Numerical Answers
1.
- N 2 O
- SiF 4
- BCl 3
- NF 3
- PBr 3
- Tl 2 Se
- NpO 2
- FeS
- CuCN
- NCl 3
- niobium (IV) oxide
- molybdenum (IV) sulfide
- tetraphosphorus decasulfide
- copper(I) oxide
- rhenium(V) fluoride
7.
a.
b.
c.
d.
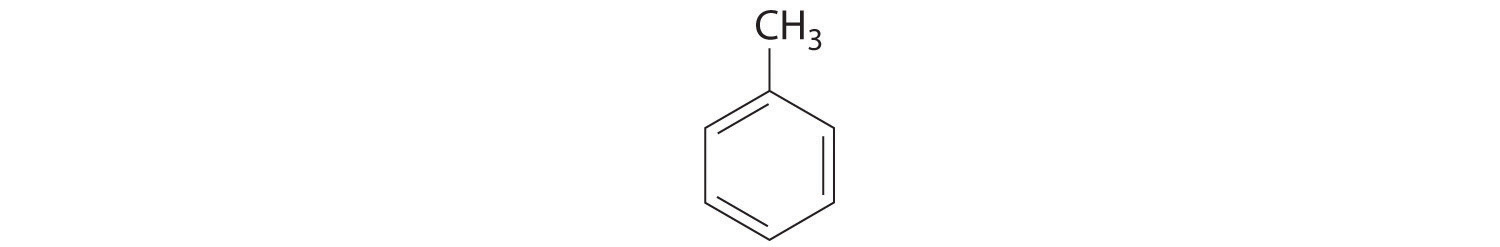
e.