Estos son ejercicios de tarea para acompañar el Mapa de texto creado para “Química: La ciencia central” por Brown et al. Se pueden encontrar bancos de preguntas de química general complementaria para otros mapas de texto y se puede acceder aquí . Además de estas preguntas disponibles públicamente, el acceso al banco privado de problemas para su uso en exámenes y tareas está disponible para los profesores solo de manera individual; comuníquese con Delmar Larsen para obtener una cuenta con permiso de acceso.
7.1: Desarrollo de la tabla periódica
Problemas conceptuales
- A Johannes Dobereiner se le atribuye el desarrollo del concepto de tríadas químicas. ¿Cuáles de los 15 elementos del grupo esperarías para componer una tríada? ¿Esperarías que B, Al y Ga actúen como una tríada? Justifica tus respuestas.
- A pesar del hecho de que Dobereiner, Newlands, Meyer y Mendeleev contribuyeron al desarrollo de la tabla periódica moderna, a Mendeleev se le atribuye su origen. ¿Por qué la tabla periódica de Mendeleev fue aceptada tan rápidamente?
- ¿Cómo explicó la contribución de Moseley al desarrollo de la tabla periódica la ubicación de los gases nobles?
- El eka – esquema de nomenclatura ideado por Mendeleev se usó para describir elementos no descubiertos.
- Utilice este método de denominación para predecir el número atómico de eka -mercurio, eka -astatina, eka -tálio y eka [19459356 ] -hafnio.
- Usando el prefijo eka , identifica los elementos con estos números atómicos: 79, 40, 51, 117 y 121.
Problema numérico
-
Según los datos proporcionados, complete la tabla.
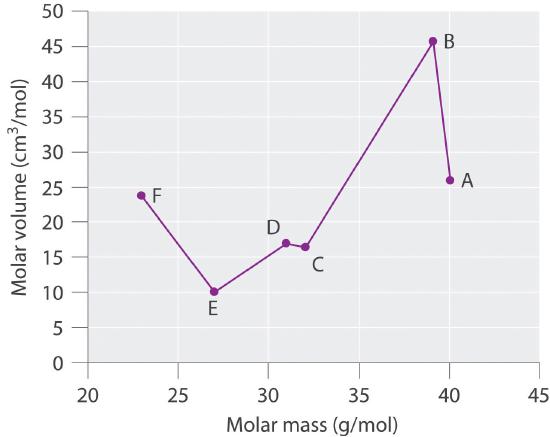
Especie Masa molar (g / mol) Densidad (g / cm3) Volumen molar (cm3 / mol) A 40.078 25,85 B 39,09 0,856 C 32.065 16,35 D 1,823 16.98 E 26,98 9,992 F 22,98 0,968 Graficar el volumen molar versus la masa molar para estas sustancias. Según Meyer, ¿cuáles se considerarían metales y cuáles se considerarían no metales?
Respuesta numérica
-
Especie Masa molar (g / mol) Densidad (g / cm 3 ) Volumen molar (cm 3 / mol) A 40.078 1,550 25,85 B 39,09 0,856 45,67 C 32.065 1.961 16,35 D 30,95 1,823 16.98 E 26,98 2.700 9,992 F 22,98 0,968 23,7 -
Meyer descubrió que los metales alcalinos tenían los volúmenes molares más altos, y que los volúmenes molares disminuían constantemente con el aumento de la masa atómica, luego se nivelaban y finalmente se elevaban nuevamente. Los elementos ubicados en la porción ascendente de una parcela de volumen molar versus masa molar eran típicamente no metales. Si observamos la gráfica de los datos en la tabla, podemos identificar inmediatamente aquellos elementos con los volúmenes molares más grandes (A, B, F) como metales ubicados en el lado izquierdo de la tabla periódica. El elemento con el volumen molar más pequeño (E) es aluminio. El gráfico muestra que los elementos subsiguientes (C, D) tienen volúmenes molares que son más grandes que los de E, pero más pequeños que los de A y B. Por lo tanto, C y D tienen más probabilidades de ser no metales (que es el caso: C = azufre, D = fósforo).
7.2: Carga nuclear efectiva
Problemas conceptuales
-
¿Qué le sucede a la energía de un orbital dado a medida que aumenta la carga nuclear Z de una especie? En un átomo multielectrónico y para una carga nuclear dada, el Z eff experimentado por un electrón depende de su valor de l . ¿Por qué?
-
La densidad de electrones de un átomo particular se divide en dos regiones generales. Nombra estas dos regiones y describe lo que cada una representa.
-
A medida que aumenta el número cuántico principal, disminuye la diferencia de energía entre niveles de energía sucesivos. ¿Por qué? ¿Qué pasaría con las configuraciones electrónicas de los metales de transición si esta disminución no ocurriera?
-
Describa la relación entre el blindaje de electrones y Z eff en los electrones más externos de un átomo. Predecir cómo la reactividad química se ve afectada por una disminución de la carga nuclear efectiva.
-
Si un átomo o ion dado tiene un solo electrón en cada una de las siguientes subcapas, ¿qué electrón es más fácil de eliminar?
- 2 s , 3 s
- 3 p , 4 d
- 2 p , 1 s
- 3 d , 4 s
7.3: Sizes of Atoms and Ions
Conceptual Problems
-
The electrons of the 1 s shell have a stronger electrostatic attraction to the nucleus than electrons in the 2 s shell. Give two reasons for this.
-
Predict whether Na or Cl has the more stable 1 s 2 shell and explain your rationale.
-
Arrange K, F, Ba, Pb, B, and I in order of decreasing atomic radius.
-
Arrange Ag, Pt, Mg, C, Cu, and Si in order of increasing atomic radius.
-
Using the periodic table, arrange Li, Ga, Ba, Cl, and Ni in order of increasing atomic radius.
-
Element M is a metal that forms compounds of the type MX 2 , MX 3 , and MX 4 , where X is a halogen. What is the expected trend in the ionic radius of M in these compounds? Arrange these compounds in order of decreasing ionic radius of M.
-
The atomic radii of Na and Cl are 190 and 79 pm, respectively, but the distance between sodium and chlorine in NaCl is 282 pm. Explain this discrepancy.
-
Are shielding effects on the atomic radius more pronounced across a row or down a group? ¿Por qué?
-
What two factors influence the size of an ion relative to the size of its parent atom? Would you expect the ionic radius of S 2− to be the same in both MgS and Na 2 S? Why or why not?
-
Arrange Br − , Al 3 + , Sr 2 + , F − , O 2− , and I − in order of increasing ionic radius.
-
Arrange P 3− , N 3− , Cl − , In 3 + , and S 2− in order of decreasing ionic radius.
-
How is an isoelectronic series different from a series of ions with the same charge? Do the cations in magnesium, strontium, and potassium sulfate form an isoelectronic series? Why or why not?
-
What isoelectronic series arises from fluorine, nitrogen, magnesium, and carbon? Arrange the ions in this series by
- increasing nuclear charge.
- increasing size.
-
What would be the charge and electron configuration of an ion formed from calcium that is isoelectronic with
- a chloride ion?
- Ar + ?
Conceptual Answers
-
The 1 s shell is closer to the nucleus and therefore experiences a greater electrostatic attraction. In addition, the electrons in the 2 s subshell are shielded by the filled 1 s 2 shell, which further decreases the electrostatic attraction to the nucleus.
-
Ba > K > Pb > I > B > F
-
The sum of the calculated atomic radii of sodium and chlorine atoms is 253 pm. The sodium cation is significantly smaller than a neutral sodium atom (102 versus 154 pm), due to the loss of the single electron in the 3 s orbital. Conversely, the chloride ion is much larger than a neutral chlorine atom (181 versus 99 pm), because the added electron results in greatly increased electron–electron repulsions within the filled n = 3 principal shell. Thus, transferring an electron from sodium to chlorine decreases the radius of sodium by about 50%, but causes the radius of chlorine to almost double. The net effect is that the distance between a sodium ion and a chloride ion in NaCl is greater than the sum of the atomic radii of the neutral atoms.
Numerical Problems
-
Plot the ionic charge versus ionic radius using the following data for Mo: Mo 3 + , 69 pm; Mo 4 + , 65 pm; and Mo 5 + , 61 pm. Then use this plot to predict the ionic radius of Mo 6 + . Is the observed trend consistent with the general trends discussed in the chapter? Why or why not?
-
Internuclear distances for selected ionic compounds are given in the following table.
-
If the ionic radius of Li + is 76 pm, what is the ionic radius of each of the anions?
LiF LiCl LiBr LiI Distance (pm) 209 257 272 296
-
-
What is the ionic radius of Na + ?
NaF NaCl NaBr NaI Distance (pm) 235 282 298 322 -
Arrange the gaseous species Mg 2 + , P 3− , Br − , S 2− , F − , and N 3− in order of increasing radius and justify your decisions.
7.4: Ionization Energy
Conceptual Problems
-
Identify each statement as either true or false and explain your reasoning.
- Ionization energies increase with atomic radius.
- Ionization energies decrease down a group.
- Ionization energies increase with an increase in the magnitude of the electron affinity.
- Ionization energies decrease diagonally across the periodic table from He to Cs.
- Ionization energies depend on electron configuration.
- Ionization energies decrease across a row.
-
Based on electronic configurations, explain why the first ionization energies of the group 16 elements are lower than those of the group 15 elements, which is contrary to the general trend.
-
The first through third ionization energies do not vary greatly across the lanthanides. ¿Por qué? How does the effective nuclear charge experienced by the n s electron change when going from left to right (with increasing atomic number) in this series?
-
Most of the first row transition metals can form at least two stable cations, for example iron(II) and iron(III). In contrast, scandium and zinc each form only a single cation, the Sc 3+ and Zn 2+ ions, respectively. Use the electron configuration of these elements to provide an explanation.
-
Of the elements Nd, Al, and Ar, which will readily form(s) +3 ions? ¿Por qué?
-
Orbital energies can reverse when an element is ionized. Of the ions B 3+ , Ga 3+ , Pr 3+ , Cr 3+ , and As 3+ , in which would you expect this reversal to occur? Explain your reasoning.
-
The periodic trends in electron affinities are not as regular as periodic trends in ionization energies, even though the processes are essentially the converse of one another. Why are there so many more exceptions to the trends in electron affinities compared to ionization energies?
-
Elements lying on a lower right to upper left diagonal line cannot be arranged in order of increasing electronegativity according to where they occur in the periodic table. ¿Por qué?
-
Why do ionic compounds form, if energy is required to form gaseous cations?
-
Why is Pauling’s definition of electronegativity considered to be somewhat limited?
-
Based on their positions in the periodic table, arrange Sb, O, P, Mo, K, and H in order of increasing electronegativity.
-
Based on their positions in the periodic table, arrange V, F, B, In, Na, and S in order of decreasing electronegativity.
Conceptual Answers
5. Both Al and Nd will form a cation with a +3 charge. Aluminum is in Group 13, and loss of all three valence electrons will produce the Al 3+ ion with a noble gas configuration. Neodymium is a lanthanide, and all of the lanthanides tend to form +3 ions because the ionization potentials do not vary greatly across the row, and a +3 charge can be achieved with many oxidants.
11. K < Mo ≈ Sb < P ≈ H < O
Numerical Problems
-
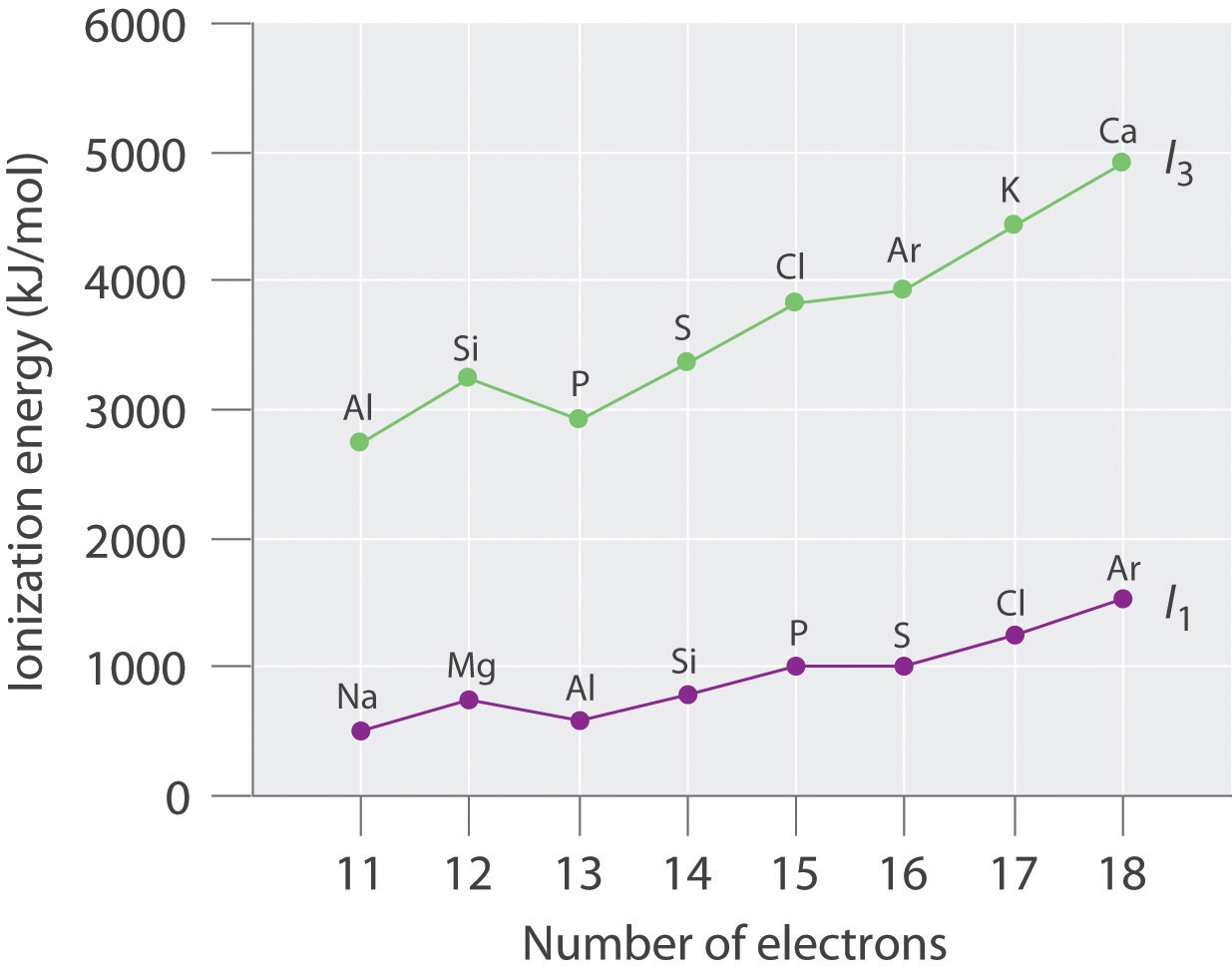
The following table gives values of the first and third ionization energies for selected elements:
Number of Electrons Element I 1 (E → E + + e − , kJ/mol) Element I 3 (E 2+ → E 3+ + e − , kJ/mol) 11 Na 495.9 Al 2744.8 12 Mg 737.8 Si 3231.6 13 Al 577.6 P 2914.1 14 Si 786.6 S 3357 15 P 1011.9 Cl 3822 16 S 999.6 Ar 3931 17 Cl 1251.2 K 4419.6 18 Ar 1520.6 Ca 4912.4 Plot the ionization energies versus number of electrons. Explain why the slopes of the I 1 and I 3 plots are different, even though the species in each row of the table have the same electron configurations.
-
Would you expect the third ionization energy of iron, corresponding to the removal of an electron from a gaseous Fe 2+ ion, to be larger or smaller than the fourth ionization energy, corresponding to removal of an electron from a gaseous Fe 3+ ion? ¿Por qué? How would these ionization energies compare to the first ionization energy of Ca?
-
Which would you expect to have the highest first ionization energy: Mg, Al, or Si? Which would you expect to have the highest third ionization energy. ¿Por qué?
-
Use the values of the first ionization energies given in Figure 7.11 to construct plots of first ionization energy versus atomic number for (a) boron through oxygen in the second period; and (b) oxygen through tellurium in group 16. Which plot shows more variation? Explain the reason for the variation in first ionization energies for this group of elements.
-
Arrange Ga, In, and Zn in order of increasing first ionization energies. Would the order be the same for second and third ionization energies? Explain your reasoning.
-
Arrange each set of elements in order of increasing magnitude of electron affinity.
- Pb, Bi, and Te
- Na, K, and Rb
- P, C, and Ge
-
Arrange each set of elements in order of decreasing magnitude of electron affinity.
- As, Bi, and N
- O, F, and Ar
- Cs, Ba, and Rb
-
Of the species F, O − , Al 3+ , and Li + , which has the highest electron affinity? Explain your reasoning.
-
Of the species O − , N 2− , Hg 2+ , and H + , which has the highest electron affinity? Which has the lowest electron affinity? Justify your answers.
-
The Mulliken electronegativity of element A is 542 kJ/mol. If the electron affinity of A is −72 kJ/mol, what is the first ionization energy of element A? Use the data in the following table as a guideline to decide if A is a metal, a nonmetal, or a semimetal. If 1 g of A contains 4.85 × 10 21 molecules, what is the identity of element A?
Na Al Si S Cl EA (kJ/mol) −59.6 −41.8 −134.1 −200.4 −348.6 I (kJ/mol) 495.8 577.5 786.5 999.6 1251.2 -
Based on their valence electron configurations, classify the following elements as either electrical insulators, electrical conductors, or substances with intermediate conductivity: S, Ba, Fe, Al, Te, Be, O, C, P, Sc, W, Na, B, and Rb.
-
Using the data in Problem 10, what conclusions can you draw with regard to the relationship between electronegativity and electrical properties? Estimate the approximate electronegativity of a pure element that is very dense, lustrous, and malleable.
-
Of the elements Al, Mg, O 2 , Ti, I 2 , and H 2 , which, if any, would you expect to be a good reductant? Explain your reasoning.
-
Of the elements Zn, B, Li, Se, Co, and Br 2 , which if any, would you expect to be a good oxidant? Explain your reasoning.
-
Determine whether each species is a good oxidant, a good reductant, or neither.
- Ba
- Mo
- Al
- Ni
- O 2
- Xe
-
Determine whether each species is a good oxidant, a good reductant, or neither.
- Ir
- Cs
- Be
- B
- N
- Po
- Ne
-
Of the species I 2 , O − , Zn, Sn 2+ , and K + , choose which you would expect to be a good oxidant. Then justify your answer.
-
Based on the valence electron configuration of the noble gases, would you expect them to have positive or negative electron affinities? What does this imply about their most likely oxidation states? their reactivity?
Numerical Answers
-
The general features of both plots are roughly the same, with a small peak at 12 electrons and an essentially level region from 15–16 electrons. The slope of the I 3 plot is about twice as large as the slope of the I 1 plot, however, because the I 3 values correspond to removing an electron from an ion with a +2 charge rather than a neutral atom. The greater charge increases the effect of the steady rise in effective nuclear charge across the row.
-
-
Electron configurations: Mg, 1 s 2 2 s 2 2 p 6 3 s 2 ; Al, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 1 ; Si, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 2 ; First ionization energies increase across the row due to a steady increase in effective nuclear charge; thus, Si has the highest first ionization energy. The third ionization energy corresponds to removal of a 3 s electron for Al and Si, but for Mg it involves removing a 2 p electron from a filled inner shell; consequently, the third ionization energy of Mg is the highest.
-
- Bi > As > N
- F > O >> Ar
- Rb > Cs > Ba
-
Hg 2+ > H + > O − > N 2− ; Hg 2+ has the highest positive charge plus a relatively low energy vacant set of orbitals (the 6 p subshell) to accommodate an added electron, giving it the greatest electron affinity; N 2− has a greater negative charge than O − , so electron–electron repulsions will cause its electron affinity to be even lower (more negative) than that of O − .
-
-
insulators: S, O, C (diamond), P; conductors: Ba, Fe, Al, C (graphite), Be, Sc, W, Na, Rb; Te and B are semimetals and semiconductors.
-
-
Mg, Al, Ti, and H 2
-
-
- reductant
- neither
- reductant
- reductant
- oxidant
- neither
-
-
I 2 is the best oxidant, with a moderately strong tendency to accept an electron to form the I − ion, with a closed shell electron configuration. O − would probably also be an oxidant, with a tendency to add an electron to form salts containing the oxide ion, O 2− . Zn and Sn 2+ are all reductants, while K + has no tendency to act as an oxidant or a reductant.
7.5: Electron Affinities
see above question to tease out
7.6: Metals, Nonmetals, and Metalloids
7.7: Group Trends fo the Active Metals
7.8: Group Trends for Selected Nonmetals